Learning Centre
A dedicated learning page for bioprocess PAT; advancing precision, efficiency, and innovation in biomanufacturing. This serves as a hub for professionals, researchers, and students to access critical information on monitoring and optimising parameters such as pH, dissolved oxygen, temperature, and nutrient levels.
By offering interactive content, real-world case studies, and up-to-date methodologies, this well-structured learning resource enhances understanding of complex bioprocess systems, fosters problem-solving skills, and accelerates advancements in biotechnology.
In an industry where accuracy directly impacts product quality and regulatory compliance, continuous learning through a dedicated platform ensures informed decision-making, reduces variability, and drives progress in sustainable and high-quality bioproduction

FDA Q2(R2) Validation of Analytical Procedures, March 2024
Q2(R2) Validation of Analytical Procedures guidance, finalised in March 2024, plays a crucial role in enhancing Process Analytical Technology (PAT) by refining the validation framework for analytical methods used in pharmaceutical and biotechnological processes. This updated guideline provides harmonised principles for validating analytical procedures, ensuring accuracy, precision, specificity, and robustness in monitoring critical quality attributes.
By establishing clear validation criteria, Q2(R2) supports real-time process monitoring, enabling manufacturers to implement advanced analytical techniques that improve efficiency and product consistency. Additionally, it aligns with risk-based approaches, allowing for flexibility in analytical method development and adaptation to evolving technology.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r2-validation-analytical-procedures
Whitepaper: A New Tool for Enhanced Process Control
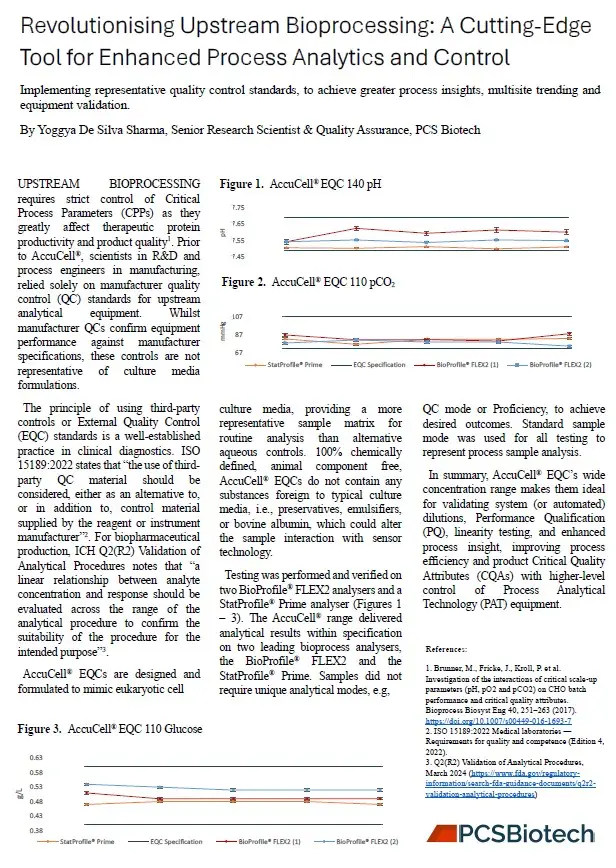
AccuCell Bioprocess EQC Standards play a vital role in process control and validation by providing a chemically defined, animal component-free reference material that closely mimics eukaryotic cell culture media. These standards ensure consistent and reliable analytical measurements, improving the accuracy of sensor technology used in bioprocess monitoring.
By offering a stable and traceable matrix, AccuCell EQCs enhance quality control (QC) and in-process control (IPC), supporting performance qualification (PQ), linearity testing, and system validation. Their sterile processing and endotoxin-free formulation make them suitable for GMP manufacturing, ensuring compliance with regulatory standards.

Copyright © 2025 PCS Biotech Limited. All content on this website is protected by copyright law | PCS Biotech Limited is registered in England and Wales with company number 13291506. VAT No. 476 1661 70 | AccuCell and ACCUCELL are registered trademarks and property of PCS Biotech Limited.
All rights reserved.